Introduction to UV Formulations
Several market forces, such as the need for increased productivity, regulatory concerns, and the need for the improved products performance, continue to push companies towards evaluating UV-curable technology as an alternative to other solvent-based, water-based, or two-part formulations. Although UV-curable formulations have many formulation parameters that are similar to other technologies, there are also factors unique to UV curing that can present a challenge to chemists new to UV.
UV-curable formulations can be cured with different curing processes such as UV-initiated curing, electron beam curing, or curing from a free-radical generating species such as a peroxide, and can refer to different chemistries, specifically free-radical curing, cationic curing, or a hybridization of the two. This series will concentrate on UV-initiated free-radical curing, as this tends to be the most common type of formulation. As a simplified explanation of UV-curing, UV-initiated free-radical curing functions by using a UV light to trigger a photoinitiator to generate unpaired electrons, called free radicals. These free radicals react with acrylated or methacrylated components in the formulation, causing them to react together in a process referred to as cross-linking that forms a polymer matrix.
To begin your formulating project, below are some of the common parameters that need to be considered:
| Formulation Parameter | Examples | Impact on Formulating Process |
|---|---|---|
| Target Application | Coating, ink, adhesive, 3D printing resin, photopolymer plate | Coatings and inks require a focus on film properties like scratch and abrasion resistance and surface energy, while applications like 3D printing resins require more focus on bulk properties of the cured material such as tensile strength and elongation. |
| Method of Application | Coatings - spray, roll coat, flow coat Inks – Flexo, offset, screen, inkjet Adhesives - precision dispense, microdot, 3D printing resin - material jetting, vat photopolymerization | The application method will dictate certain characteristics of the formula apart from its cured properties, such as its viscosity, rheology, surface energy/contact angle, and compatibility with mechanical components that the uncured resin may be in contact with. |
| Substrate | Wood, vinyl, polycarbonate, stainless steel, aluminum, glass, polypropylene, PMMA, PET | Proper raw material selection is crucial to the adhesion characteristics of your formulation, and adhesion of the formulation will vary widely based on the substrate. Free-radical curing formulations tend to have challenges adhering to metals, and inert plastics such as high-density polyethylene (HDPE) tend to be very challenging substrates for adhesion. |
| Curing Conditions | Broad spectrum mercury, metal halide, or visible light sources; LED units at specific wavelengths (405, 395, 385, 365 nm); spot vs. flood vs. conveyor vs. roll-to-roll application; high intensity vs low intensity; desired line speeds | Cure conditions will impact photoinitiator selection and weight ratio, the functionality of monomers and oligomers, and the ability to use different pigments or UV absorbers. |
| Viscosity Requirements | Low viscosities (<100 cP) for inkjet inks and thin coatings; medium viscosities (100-5,000 cP) for 3D printing resins, nail coatings, and medium build coatings; high viscosities (>5,000 cP) for adhesives, offset inks, and thixotropic coatings | Low viscosity requirements will limit your ability to use high viscosity or high molecular weight materials and will require the use of high quantities of monomers; high viscosity and thixotropy requirements may require the use of different additives in order to build the proper rheological profile of the formulation. |
| Stain, Chemical, And Thermal Resistance | Moisture, high temperatures, extremely low temperatures, outdoor exposure, household chemicals, fuels and oils, acids or bases, food products | Raw material selection will vary widely based on what types of chemistries or environments that the cured product will be exposed to. Increasing the crosslink density of your formula tends to improve resistance to aggressive environments. |
| Surface Effects | Soft feel, high gloss vs. matte, slip resistant vs. low friction, tacky vs. tack free, hydrophobic vs. hydrophilic, clear vs. pigmented | Different additive packages and backbone chemistries can be used to alter the feel or surface characteristics of the formula. Waxes and particles can be used to impart a variety of surface feels or visual effects. |
| Regulatory Needs | Food contact (FDA or Swiss List compliance), medical device extractables standards, VOC thresholds, country chemical inventory registrations | Even if your formulation works properly, if the materials are not compliant with application-specific regulations or are not listed on the appropriate chemical inventories (e.g., TSCA, REACH, etc.), the formulation will not be a viable commercial product. |
Once the above parameters are reviewed and understood, it is time to formulate. UV formulations involve four primary materials:
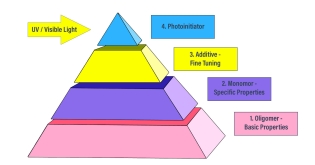
- Oligomers – the foundation of your formula
- Monomers – reactive diluents to lower viscosity and modify some of the oligomer properties
- Additives – to fine tune properties or add properties not available in oligomers or monomers
- Photoinitiators – to absorb UV light and create free-radicals to crosslink the oligomers and monomers
These ingredients, when crosslinked, work together to build the UV-cure product. Other Bomar blog posts in this series explore these building blocks and explain what factors to consider for each when designing your UV-curable formulation.