Formulation Overview: Photoinitiators
The final step of the formulation process is the addition of photoinitiators. Photoinitiators absorb light at different wavelengths and form free radicals that initiate crosslinking and the curing of a formula.
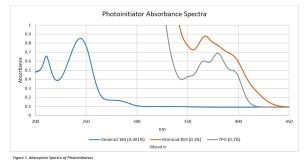
Figure 1 represents a diagram of absorbance curves of four different photoinitiators: Omnirad 2959, Darocur 1173, Irgacure 184, and Irgacure 754. The wavelengths of light emitted by the curing lamp determine which photoinitiator best suits a formulation. Photoinitiator suppliers provide absorbance curves to aid in this determination.
Photoinitiators can be supplemented with compounds like photosensitizers, amines, thiols, and co-initiators that enhance its properties. These materials, used to modify the cure of a formula, are the reason that this ingredient of the formula is known as a photoinitiator package.
It is necessary for photoinitiator concentration to be optimized when considering a formula. Not enough photoinitiator can result in slow and incomplete cure while too much photoinitiator will block UV light and result in poor through cure. Thinner films require an increase in photoinitiator concentration and ultra-thin coatings (2-5µ) may require a high concentration of photoinitiator to thoroughly cure. The most effective photoinitiator package can be determined by running ladder studies.
Two Types of Photoinitiators
For free-radical curing, there are two basic types of photoinitiators: Norrish Type I and Norrish Type II. Type I photoinitiators cleave when excited by UV light resulting in a free-radical. When Type II photoinitiators are excited, they abstract a hydrogen or electron from a donor (usually an amine). The extra electron produces a free-radical that initiates the reaction. Examples of Type I photoinitiators are alpha hydroxy ketones and phosphine oxides. Examples of Type II photoinitiators are benzophenones and thioxanthones.
Sensitizers
Sensitizers are used to extend the wavelengths that photoinitiators absorb. The sensitizers will absorb the UV energy and transfer the energy to the photoinitiator, then return to its ground state.
Amine Synergists
There is a synergistic effect between amines and Type II photoinitiators. Amines act as hydrogen donors for Type II photoinitiators.
Through Cure
For through cure, photoinitiators that absorb longer wavelengths (350 to >400 nm) generally are more successful. Longer wavelengths will penetrate deeper than shorter wavelengths. For these photoinitiators to work, the light source must emit in the absorption range of the photoinitiator.
Surface Cure
In general, photoinitiators that absorb in the shorter wavelengths (200 to 300 nm) are better at surface curing. Therefore, if using a 405 nm LED light source, adding a short wavelength photoinitiator would not be very effective because the lamp will not emit short wavelengths. Surface cure can be achieved by adding high functional materials such as monomers and oligomers. Certain blends of photoinitiators can also increase the cure rate, resulting in higher cure speeds. Thiols can increase the speed of the reaction, which helps to negate the oxygen inhibition.
Interference Compounds
Additives such as fillers, pigments, UV stabilizers and excessive photoinitiator in the formula can interfere with light absorption. Different photoinitiators are designed to work with different colored pigments because some pigmented materials can be troublesome regarding through cure. Interference can also stem from the thickness of the coating. Thicker coatings absorb light and may not be able to achieve full thickness.
When Formulating with Photoinitiators
- Make sure the wavelengths align between photoinitiator and light source.
- Use aids like synergists or sensitizers to help with utilizing more of the light energy.
- Avoid issues with interference. Use photoinitiators designed for the pigments you are using.
- A reduction in free radicals can lead to poor surface cure, poor physicals and poor over all cure.