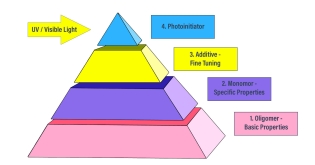
Formulation Overview: Monomers
Oligomers represent the basic properties of a formula while monomers are used to continue to enhance or dilute those properties. In the next step of the formulation process, monomers can help to narrow oligomer characteristics even further to create a more specific formula development. Just as water or solvents are used in conventional systems, monomers are reactive diluents that lower viscosity and improve the flow of a formula. Because they are non-volatile and reactive, monomers do not need to be evaporated from the coating as they crosslink to the cured resin.
Several different types of monomers allow for specific modification to the already present properties brought by the base oligomer of the formula. Functionality plays a large role in choosing a monomer and can range from monofunctional to as much as pentafunctional. Most monomers are mono or difunctional however, trifunctional monomers are also often used. Low functional monomers generally lower viscosity, offer better adhesion, and increase flexibility of a formula. Due to their structure, monofunctional materials chain extend rather than crosslink contributing to flexibility while difunctional materials crosslink causing more rigidity. With these lower functional monomers, because of the lower acrylate concentration there is a risk of lower cure speeds and residual uncured product. High functional monomers cause crosslinking rather than chain extension which can improve scratch resistance, lower flexibility, increase exotherm, and yield less adhesion to substrates. With higher acrylate concentration, they exhibit faster cure speeds, less residual uncured product.
Monomer chemistry is a large aspect of the enhancing or dilution of properties in a formula. For example, long chain molecules will impart more flexibility, but may also be higher in viscosity. Highly ethoxylated monomers may impart water solubility and hydrophilic properties. Examples of monomers that are generally very good solvents include: hexanediol diacrylate (HDDA), hydroxyethyl methacrylate (HEMA), tetrahydrofurfuryl methacrylate (THFMA), and 2-(2-ethoxyethoxy)ethyl acrylate (EOEOEA). These monomers help to efficiently dissolve solids, like photoinitiators, into a formula. Monomer package selection is important when adjusting properties in a formulation such as surface tension, adhesion to certain substrates, chemical resistance, and a variety of other application parameters.
Some monomers are used solely to impart properties on a formulation however, more than one monomer can be used as part of a monomer package in a formula. For example, dipentaerythritol pentaacrylate can be used to impart increased reactivity to a sluggish recipe that may already include another monomer. It is not used to lower the viscosity as the viscosity is around 10,000 cps. The key to choosing a monomer or monomer package is to balance the desired viscosity with the other property requirements.
Surface tension as it relates to hydrophobicity
- Behenyl acrylate – low surface tension
- Neopentyl glycol – not super hydrophobic but has a low surface tension
- Steryl – hydrophobic
- C12 alkyl methacrylate – very hydrophobic
- IDA
- Trimethyl cyclohexyl acrylate – very hydrophobic; surface tension of 27.3 (SR-420)
- IOA